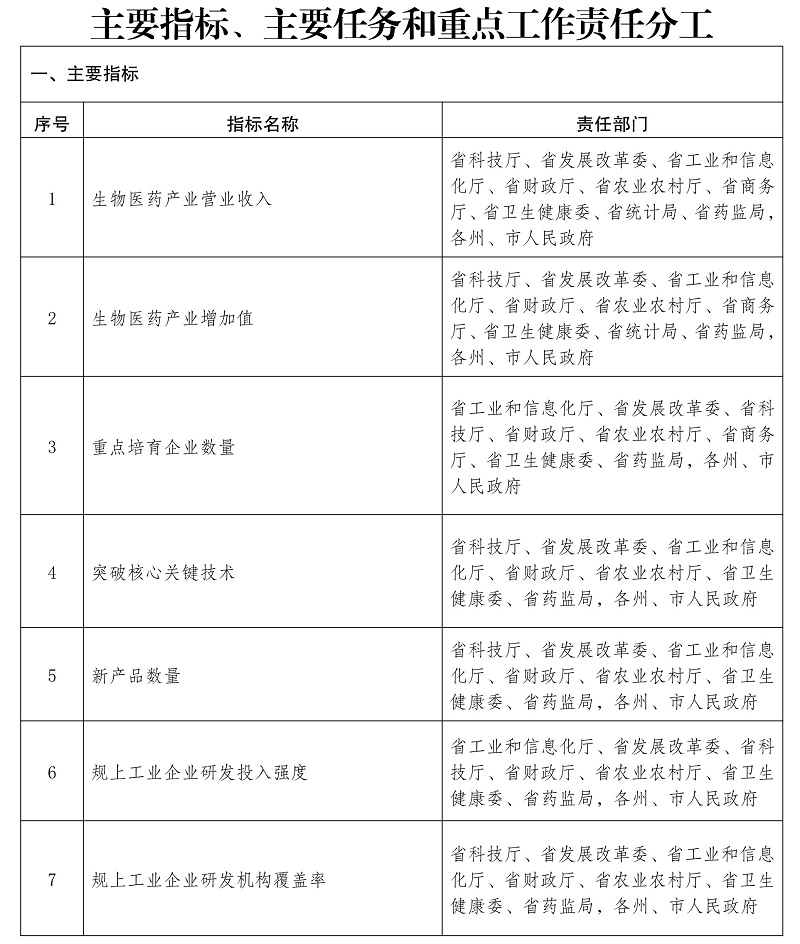
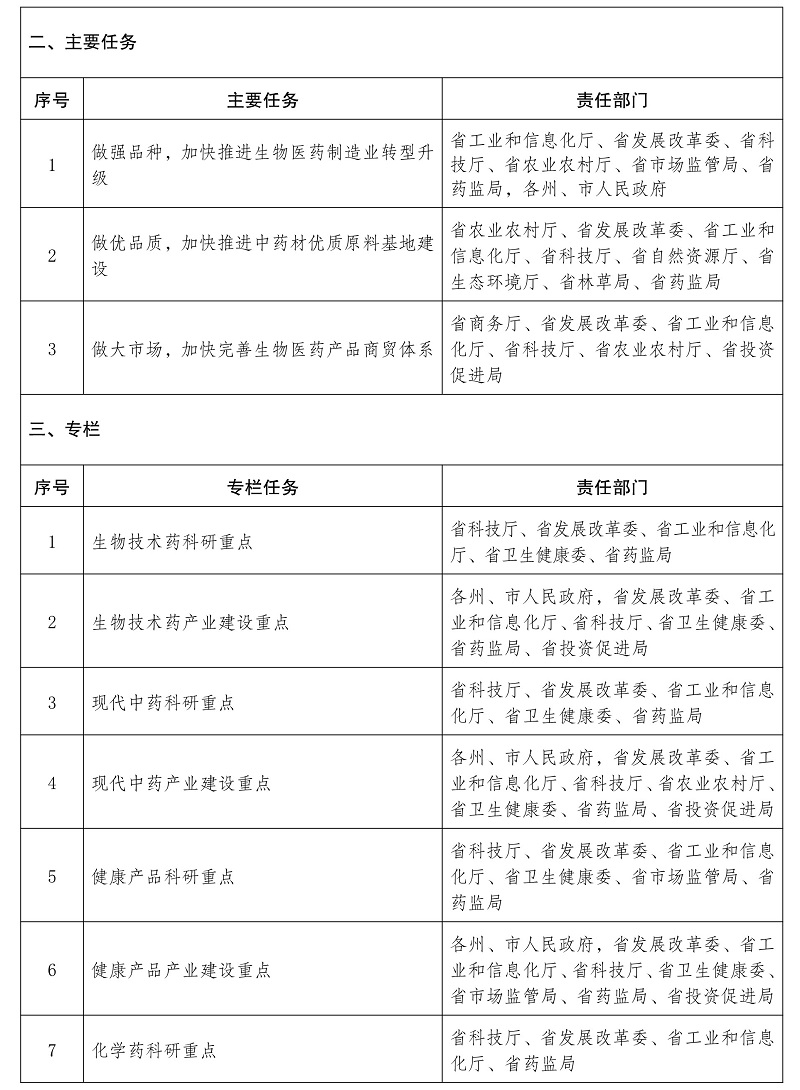
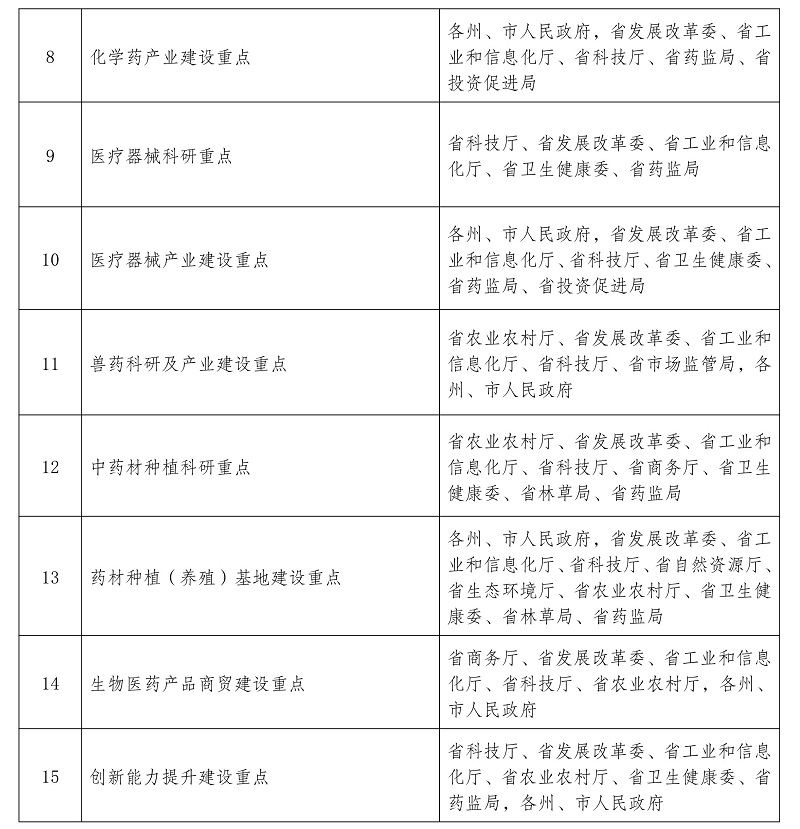
Notice of the General Office of the People’s Government of Yunnan Province on Printing and Distributing the Innovative Development Plan of Biomedical Industry in Yunnan Province during the 14th Five-Y
State and municipal people’s governments, provincial committees, offices, departments and bureaus:
"Yunnan Province" 14 th Five-Year Plan "for the Innovation and Development of Biomedical Industry has been agreed by the provincial people’s government and is hereby issued to you, please implement it carefully.
General Office of Yunnan Provincial People’s Government
March 17, 2022
(This piece is publicly released)




In order to accelerate the innovation and development of the biomedical industry, this plan is formulated according to the 14th Five-Year Plan for National Economic and Social Development in Yunnan Province and the Outline of Long-term Objectives for the Year 2035, combined with the reality of our province.
I. Development Status and Situation
(A) Development effectiveness
During the "Thirteenth Five-Year Plan" period, the provincial party committee and the provincial government conscientiously implemented the spirit of the important speech of the Supreme Leader General Secretary on his inspection of Yunnan, and on the basis of continuously building eight key industries and world-class "three cards", put forward the strategic deployment of focusing on cultivating five trillion-level pillar industries and eight billion-level advantageous industries and building a modern industrial system. Biomedical industry, as a strategic emerging industry with a focus of 100 billion yuan in our province, has steadily expanded its scale, significantly enhanced its innovation ability, increasingly optimized its policy environment, and continuously improved its economic benefits. Its operating income increased from 124.109 billion yuan in 2015 to 252.145 billion yuan in 2020, with an average annual growth rate of 15.23%, and its added value increased from 36.587 billion yuan in 2015 to 73.14 billion yuan in 2020. The average annual growth rate reached 14.86%, the business income and added value doubled, and a research and production system of biotechnological drugs focusing on vaccines was gradually formed, and a whole industrial chain development system of natural drugs (ethnic Chinese medicines) featuring proprietary Chinese medicines, Chinese herbal pieces, extracts and health products was established.
In 2020, the province’s Chinese herbal medicine planting (breeding) industry will realize an operating income of 51.611 billion yuan; The biomedical manufacturing industry realized an operating income of 59.592 billion yuan; The biomedical commerce and trade industry realized an operating income of 140.942 billion yuan. There are 163 pharmaceutical manufacturing enterprises above designated size (including 1 enterprise with operating income exceeding 10 billion yuan and 5 enterprises with 1-10 billion yuan). There are 48 varieties with sales income exceeding 100 million yuan (including 5 varieties exceeding 1 billion yuan and 4 varieties with 500-1 billion yuan). Up to now, there are 9 biomedical enterprises listed on the main board and growth enterprise market in the province.
1. The biotechnology pharmaceutical industry has made breakthrough development.
In 2020, the province’s biological products manufacturing industry above designated size represented by vaccines achieved an operating income of 5.357 billion yuan, accounting for 15.6% of the operating income of pharmaceutical manufacturing industry above designated size. There are 13 vaccine varieties on the market, and the operating income of vaccine enterprises has increased from 800 million yuan in 2009 to 4.8 billion yuan in 2020, and the biotechnology drug industry has made breakthrough progress. The inactivated enterovirus 71 vaccine independently developed by the Institute of Medical Biology, Chinese Academy of Medical Sciences was launched in December 2015, and the annual operating income of a single variety was nearly 2 billion yuan. The 23-valent pneumococcal polysaccharide vaccine developed by watson biological was launched in July 2017, with an operating income of 688 million yuan in 2020; The first trivalent pneumococcal polysaccharide conjugate vaccine in China and the second trivalent pneumococcal polysaccharide conjugate vaccine in the world went on the market in April 2020, with an operating income of 1.658 billion yuan in the first year, and the market prospect is considerable. Some traditional pharmaceutical companies and scientific research units have accelerated the layout and development of biotechnological drugs. Suparutide injection, a long-acting hypoglycemic drug developed by Kunyao Group, has obtained clinical approval, and Sino Pharmaceutical has carried out research on new drugs of humanized CD22 antibody immunotoxin 1.1.
2 Chinese medicine (ethnic medicine) industry scale
In recent years, the traditional Chinese medicine (ethnic medicine) industry in our province has become the main force to promote the development of biomedical industry from small to large and from weak to strong. 60 "Hometowns of Yunyao" and 103 "Pharmaceutical Parks" have become the new force to improve the quality and brand of Chinese herbal medicines. In 2020, the main business income of planting (breeding) and product processing of Chinese herbal medicines in the province will exceed 100 billion yuan, among which the processing and manufacturing of Chinese herbal medicines above designated size, such as proprietary Chinese medicines, Chinese herbal pieces, extracts and health products, will achieve an operating income of about 48 billion yuan, accounting for over 80% of the main business income of biomedical industries above designated size. The planting area of Chinese herbal medicines exceeds 9 million mu, the planting area of 15 varieties of Chinese herbal medicines, such as Panax notoginseng, Paris polyphylla, Dendrobium, Amomum villosum and Radix Aucklandiae, exceeds 100,000 mu, and the standardized planting base of Chinese herbal medicines covers an area of 1.46 million mu, including 22,000 mu of green certification base and 126,000 mu of organic certification base. Sanqi, Erigeron breviscapus industry has formed a whole industrial chain layout of planting, processing, R&D and production, which has played a very prominent role in demonstrating the development of traditional Chinese medicine industry in the province. Panax notoginseng industry has achieved standardization and standardized planting, and developed relatively complete back-end products such as decoction pieces, preparations and health products, which has become a big variety second only to ginseng. The planting area of the whole province is about 400,000 mu, and the agricultural output value is about 6 billion yuan. A number of Chinese patent medicines and injections such as Panax notoginseng decoction pieces, Xuesaitong soft capsules, Xuesaitong for injection (freeze-dried powder injection), Xuesaitong tablets, Xuesaitong capsules, Panax notoginseng saponins and Xuesaitong injection, etc.
3. The chemical medicine industry has developed steadily.
In 2020, the chemical preparation manufacturing industry and the chemical raw material medicine manufacturing industry above designated size will realize an operating income of 3.52 billion yuan, accounting for 10.2% of the pharmaceutical manufacturing industry above designated size. Initially gathered a number of chemical pharmaceutical enterprises such as Jida Pharmaceutical, Baker Norton, Haobang Pharmaceutical, Sino Pharmaceutical, Longhai Pharmaceutical and Guiyan Pharmaceutical; Longjin Pharmaceutical, Haobang Pharmaceutical and Plant Pharmaceutical cooperated with Indian generic drug companies to actively introduce Indian generic drug varieties to Yunnan; Nine varieties, including amoxicillin capsules, chlorphenamine maleate tablets and ambroxol hydrochloride injection, passed the consistency evaluation of chemical generic drugs.
4. Remarkable achievements have been made in biomedical science and technology innovation.
Driven by scientific and technological innovation, the biomedical industry has gradually embarked on a high-quality development track. Sabin inactivated poliomyelitis vaccine, EV71 inactivated enterovirus vaccine, 13-valent pneumococcal polysaccharide conjugate vaccine and other fist products have been listed one after another, and some varieties are undergoing WHO pre-certification; Covid-19 inactivated pneumonia vaccine, F gene mumps live attenuated vaccine, nine-valent human papillomavirus (HPV) vaccine, rotavirus inactivated vaccine and tetravalent meningococcal polysaccharide conjugate vaccine are in the clinical research stage, which has provided strong support for the rapid development of the vaccine industry during the Tenth Five-Year Plan. Ten innovative drugs, such as KPCXM18, a natural medicine for treating ischemic stroke, Duanjin Jiedu Capsule, a 6-class Chinese medicine, Suparutide injection, and a 1.1-class new drug of bungarus multicinctus venom, were approved by the national drug clinical approval. Winona series products, with authentic Yunnan medicinal materials as the main raw materials, have developed into an important functional skin care brand in China. "Key Technology Creation and Industrial Application of Comprehensive Development of Panax notoginseng" won the second prize of National Science and Technology Progress Award, "Key Technology and Application of Standardization and Industrial Development of Panax notoginseng" won the first prize of Yunnan Science and Technology Progress Award, and three standards of Panax notoginseng, seeds and seedlings and Gastrodia elata passed the international quality standard certification. Panax notoginseng saponins and breviscapine have passed GRAS (generally considered safe) certification recognized by FDA, and entered the catalogue of American botanical medicine raw materials;Tongshu capsule and Xuesaitong soft capsule were approved by FDA to carry out phase II clinical research.
Around the great demand of the development of biomedical industry, a number of R&D service platforms have been built, such as Yunnan Biovaccine Technology Innovation Center, Yunnan Drug Non-clinical Safety Evaluation Research Center, Yunnan Natural Drug Activity Screening and Evaluation Center, Yunnan Stem Cell and Regenerative Medicine Research Center, Yunnan Flower Aromatic Health Products R&D Center, and Yunnan Chinese Medicine Seed Breeding R&D Center. By the end of the 13th Five-Year Plan, there were 47 provincial key laboratories in the biomedical field, 20 engineering technology research centers, 19 engineering research centers, 10 clinical medical research centers and 4 academicians of the two academies in the province, and 12 provincial science and technology leaders were trained, 54 provincial high-level talents were introduced, 4 high-level innovation and entrepreneurship teams were introduced, 553 provincial young and middle-aged academic and technological leaders and 553 provincial innovative talents were trained.
5. Bio-pharmaceutical industry has achieved initial results by attracting big and strong players.
Yunnan Baiyao Group introduced social capital to complete the mixed reform, and the development of the group entered the fast lane; China Resources Sanjiu acquired Kunming Shenghuo Pharmaceutical, and the company’s operating income increased significantly; Guizhou Bailing invested in Yunnan plant pharmaceutical industry, focusing on characteristic products, optimizing product structure, and implementing large-variety brand strategy. The scale of the enterprise has maintained rapid growth for many years. Well-known enterprises inside and outside the province, such as Sinopharm Group, Shanghai Pharmaceutical Group, Guangzhou Pharmaceutical Group, Tasly Pharmaceutical, Kangenbei Pharmaceutical, Shenwei Pharmaceutical, New Green Pharmaceutical, Beijing Tongying, Fangsheng Pharmaceutical, Jilin Xingshen, Hong Kong Juzhang and Shenzhen Botton, have landed in Yunnan and actively participated in the healthy development of the biomedical industry.
(B) Advantages and shortcomings
1. Location advantage
Yunnan has outstanding geographical advantages. It is adjacent to Guizhou and Guangxi in the east, Sichuan in the north, Tibet in the northwest, and Myanmar, Laos and Vietnam in the west and south. It is an important channel connecting South Asia, Southeast Asia and the Indian Ocean region, and an important intersection of the "Belt and Road" construction and the national development strategy of the Yangtze River Economic Belt. The biomedical industry has a huge potential market, which can be built on the basis of vaccines and traditional Chinese medicine (ethnic medicine)
2. Resource advantages
Yunnan has obvious three-dimensional climate characteristics, many types, beautiful mountains and rivers, pleasant climate and outstanding biodiversity characteristics. It is known as the "plant kingdom", "animal kingdom", "microbial kingdom", "hometown of medicinal materials" and "treasure house of biological genes", and is the province with the richest biological resources, natural medicines and ethnic medicine resources in China. According to the statistics of the third national survey of traditional Chinese medicine resources, there are 6,559 kinds of traditional Chinese medicine resources in the province, accounting for 51.4% of the total number of kinds in the country, more than 2,000 kinds of ethnic medicine resources and more than 10,000 folk prescriptions.
3. Advantages of industrial base
Notoginseng Radix, Rhizoma Paridis Yunnanensis, Erigeron breviscapus, Dendrobium candidum, Fructus Amomi, Rhizoma Gastrodiae, Poria Yunensis, Radix Angelicae Sinensis, Radix Aucklandiae, Radix Gentianae Yunnanensis and other "Top Ten Yunyao" brand medicinal materials have excellent quality and are widely recognized in the industry. It has built a domestic raw material base for high-quality natural medicines and health products. The large varieties of Yi medicine represented by Yunnan Baiyao, the unique ethnic medicines such as Dai and Naxi, and the unique ethnic medical technologies such as Dai medicine, which are in the same strain as the traditional medical systems of neighboring countries, provide rich ethnic medical resources for the innovation, inheritance and development of traditional Chinese medicine and the opening up of markets for South Asian and Southeast Asian countries. Gu Fangzhou, known as the "father of polio vaccine in China", engaged in the research of live attenuated polio vaccine in Yunnan, established the method of isolation and finalization of polio virus, formulated the trial production and safety standards of live polio vaccine, and formulated the "Rules for the manufacture and verification of live polio vaccine", which guided the production and identification of billions of vaccines in China and laid a solid foundation for the rapid development of vaccine industry in our province.
4. Shortcomings of rapid industrial development
First, the industry is small in scale and weak in competitiveness. The overall scale of Yunnan’s biomedical industry is small. Among the top 500 main business incomes of the national pharmaceutical industry, there are only 10 in Yunnan. The overall competitiveness of the biomedical industry, which is dominated by traditional Chinese medicine, is weak, especially affected by the national medical reform policies such as the catalogue of auxiliary drugs of traditional Chinese medicine, the catalogue of medical insurance, and the consistency evaluation of traditional Chinese medicine injections. The scale of biomedical manufacturing industry has declined slightly for two consecutive years.
Second, the industrial structure is not excellent and the industrial chain is incomplete. The development of standardized planting (breeding) bases of Chinese herbal medicines lags behind, the phenomenon of small varieties of Chinese herbal medicines is prominent, the research foundation is uneven, and the competitiveness of products is generally weak; Vaccines are the main biotechnological drugs, but the pipelines of antibody drugs, biological similar drugs, cell products, blood products and other products are not rich, and the upstream and downstream supporting industries are basically blank; Small amount of chemicals, lack of original research drugs and high-end generic drugs; The development level of medical devices and veterinary drugs is low.
Third, the core competitiveness of enterprises is weak, and the degree of specialization of the park is low. There are few well-known enterprises and leading enterprises, leading enterprises are not aware of advanced layout and innovative R&D activities, and the industry is not leading enough; Insufficient investment in research and development, lack of influential and innovative varieties, and weak core competitiveness; The level of professional development of biomedical industrial park is not high, the supporting system is not perfect, and the public R&D and service capabilities are weak.
Fourth, innovation ability is not strong, and high-level talents are lacking. The research and development system of new drug creation is imperfect, the resources are scattered, and the technical level is not high, which does not match the needs of the industrial chain; Lack of forward-looking scientific research ability for common technologies, key technologies and cutting-edge technologies needed for rapid industrial development; There are few innovative products in other industries except vaccines; The linkage between enterprises and scientific research institutions is not close, and the contradiction between supply and demand in the transformation of scientific and technological achievements is prominent; Lack of high-level R&D innovation centers and insufficient public R&D service platforms such as drug safety evaluation (GLP), drug clinical evaluation (GCP) and contract R&D institutions (CRO); There is a serious shortage of high-level innovative talents, management talents and teams.
Fifth, brand building is not enough, and market development ability is weak. Enterprises are weak in coping with industrial policies such as medical reform, drug review and approval, and drug production supervision, and weak in market cultivation and brand building of their own products; There is a lack of systematic publicity highlighting the advantages and characteristics, insufficient excavation of superior Chinese herbal medicine varieties, time-honored traditional Chinese medicine and ethnic medicine culture, and weak brand building and market promotion of "Yunyao".
(3) Opportunities and challenges
1. Health needs are increasing.
With the increase of sub-health population and the acceleration of population aging, people’s demand for health products has increased rapidly; The demand for health consumption has changed from medical treatment to disease prevention, health care and health promotion; The national new medical reform plan puts the prevention and control of diseases in the first place, and pays more attention to "preventing diseases before they occur". Traditional Chinese medicine (ethnic medicine) will be promising.
2. Building a domestic and international dual cycle will bring new opportunities for industrial development.
The COVID-19 epidemic has had a great impact on the global industrial chain and supply chain. It is an inevitable requirement to maintain a stable and healthy economic development by adjusting the economic development path, opening up the international circulation, smoothing the domestic circulation, coordinating development and security, enhancing the autonomy, sustainability and resilience of economic development. Seize the development opportunities brought by the Regional Comprehensive Economic Partnership Agreement (RCEP), make full use of Yunnan’s geographical advantages in connecting South Asia, Southeast Asia and the Indian Ocean region, and exchange the traditional medicine with neighboring countries, realize the smooth connection with the biomedical industry in South Asia and Southeast Asia, and accelerate the construction of a new international and domestic dual-cycle development pattern.
3. The epidemic situation in COVID-19 affected the development pattern of pharmaceutical industry.
After the outbreak of COVID-19, COVID-19’s vaccine research and development and production have become the focus of international competition in the field of biotechnology drugs, which puts forward new requirements for the corresponding research and development technology system, experimental evaluation, manufacturing capacity level and organizational management efficiency. With the development of modern biotechnology such as genetic engineering, cell engineering, tissue engineering, biosynthesis, bioinformatics and precision medicine, biotechnological drugs such as vaccines and antibody drugs have become the main direction of the transformation and upgrading of the biomedical industry. The competitive advantages of prescription and medicine represented by "three drugs and three parties" are prominent, and the modern innovative Chinese medicine with clear mechanism of action and definite clinical effect has obvious competitiveness. The demand for medical materials represented by ventilators, testing reagents, medical masks and protective clothing has surged, which has promoted the development of the medical device industry.
4. Competition in domestic pharmaceutical industry is becoming increasingly fierce.
All provinces (autonomous regions and municipalities) take biomedicine as a priority industry, and Beijing, Shanghai, Jiangsu, Guangdong and other provinces (municipalities) have been at the forefront of the country by virtue of their own geographical, policy, talent and technical advantages; Shandong, Sichuan, Hubei, Jiangxi and other provinces have also accelerated the pace of industrial development, and the competition between regions has become increasingly fierce. The reorganization of enterprises in the industry is frequent, and super-large enterprises are constantly emerging. The development trend of agglomeration is obvious, full of opportunities and challenges.
5. The medical reform policy forces the pharmaceutical industry to develop with high quality.
The research and development, production and sales of pharmaceutical products are highly dependent on policy factors, and the industrial development is deeply influenced by policy regulation. The continuous adjustment of various policies, such as drug supervision, the catalogue of traditional Chinese medicine auxiliary drugs and the new medical insurance catalogue, has prompted pharmaceutical enterprises to increase investment in research and development, take a higher quality development path, change from extensive development to refined development mode, and change products from imitation to original innovation. At the same time, information technology represented by mobile medical care, cloud computing, big data and Internet of Things has begun to penetrate into all aspects of the biomedical industry.
6. Competition for innovative drugs has intensified.
At present, the research and development of major innovative drugs are still led by well-known multinational companies, and the competition for technology, talents and funds in the biomedical industry is extremely fierce. Large pharmaceutical companies focus on developing innovative drugs for clinical value in the fields of biotechnology drugs and chemical drugs, while expanding new indications of existing products and expanding upstream and downstream business chains. Pharmaceutical companies with obvious competitive advantages in core products, diversified products and outstanding R&D capabilities will have stronger competitiveness and anti-risk ability. The global vaccine industry presents an oligopoly pattern, with the top 10 companies accounting for 95.7% of the total vaccine market share, and the four vaccine giants GSK (GlaxoSmithKline), Merck, Sanofi and Pfizer accounting for about 80% of the total market share. In the future, the global vaccine market has great growth potential and will grow rapidly with a compound annual growth rate of 10%. The rapid development of frontier technologies such as gene therapy and cell therapy is expected to form major innovative drugs and new supporting technologies. The patents of the top 10 original drugs in global sales, such as Hummel, Merlot and Avastin, are about to expire, and generic drugs and bio-similar drugs are expected to usher in new opportunities. From the national situation, the major achievements of new drug research and development are mainly concentrated in Beijing, Shanghai, Jiangsu and other places, and innovative enterprises led by new drug research and development, such as Hengrui Pharma and Wuxi PharmaTech, have a strong development momentum.
Second, the development ideas, principles and objectives
(A) development ideas
Guided by the Supreme Leader’s Socialism with Chinese characteristics Thought in the New Era, we will fully implement the spirit of the 19th National Congress of the Communist Party of China and the previous plenary sessions of the 19th National Congress, thoroughly study and implement the spirit of the important speech of the Supreme Leader’s General Secretary on his visit to Yunnan, conscientiously implement the decision-making arrangements of the provincial party committee and government, grasp the new development stage, implement the new development concept, serve and integrate into the new development pattern, adhere to the problem-oriented, goal-oriented and result-oriented, take innovation as the core, quality as the foundation, and take enterprises as the main body. Market-oriented, focusing on the three key areas of biotechnological drugs, modern Chinese medicine and health products, we will focus on attracting investment accurately, strengthening the main body of enterprises, enhancing innovation ability, building industrial clusters, promoting industrial development with engineering measures, building domestic first-class R&D and production bases of biological vaccines and modern Chinese medicine, domestic top-quality raw materials bases of natural drugs and health products, and biomedical trade bases facing South Asia and Southeast Asia, forming an industrial cluster with important influence in the country, and realizing the high-quality development of Yunnan biomedical industry.
(2) Basic principles
1. Innovation-driven, quality-improved development
Around the industrial chain, deploy innovation chain, build a batch of high-level public service platforms and professional R&D platforms, break through a batch of technologies, reserve a batch of achievements, develop a batch of products and transform a batch of achievements, and promote the high-quality development of Yunnan biomedical industry.
2. Based on advantages and outstanding features
Based on the advantages of Yunnan’s location, ecology, environment, resources and nationality, we will highlight the characteristics of biological vaccines, authentic medicinal materials, ethnic medicine, classic and famous prescriptions, proven prescriptions and cell technology, strengthen product quality and standards, and accelerate industrial transformation and upgrading.
3. Government guidance and market dominance
Innovate institutional mechanisms, rationally allocate resources, break through policy bottlenecks, cultivate and expand market players, release enterprise vitality and market potential, and build a sound market system and policy system.
4. Open cooperation and push forward together.
Improve the soft and hard environment, expand the domestic and foreign markets, and support local enterprises to become bigger and stronger; Introduce well-known enterprises and scientific research achievements to expand the increment; Attracting scientific research institutes from outside the province to develop in Yunnan, jointly cultivating talents, focusing on R&D and innovation, and forming a strong synergy to promote industrial development.
(III) Overall objectives
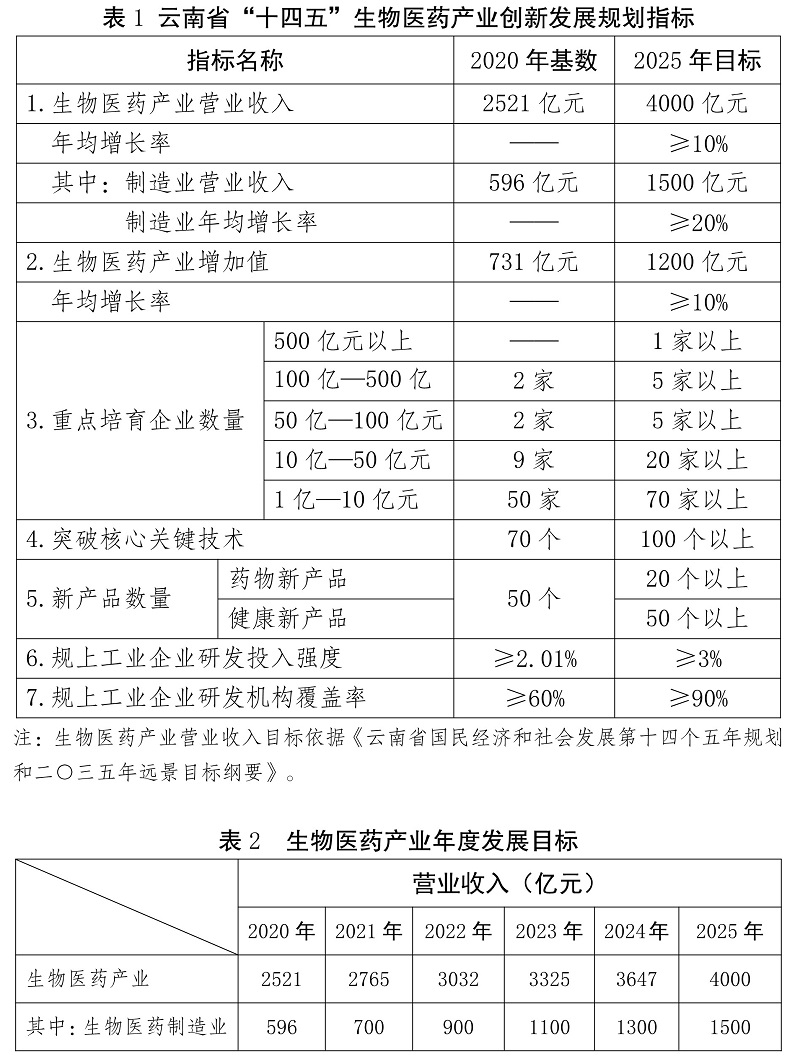
By 2025, the operating income of the biomedical industry will achieve the goal of "ensuring four guarantees and competing for five", that is, it will reach 400 billion yuan and strive to reach 500 billion yuan, with an average annual growth rate of more than 10%. Among them, the biomedical manufacturing industry will achieve an operating income of more than 150 billion yuan, with an average annual growth rate of more than 20%. The added value of biomedical industry reached 120 billion yuan, with an average annual growth rate of more than 10%. Strive for the biological vaccine industry to achieve the number and value of batch issuance. Facing 2035, we will focus on key markets, increase promotion, actively tap the domestic market, vigorously explore the South Asia and Southeast Asia markets, further enhance the influence and market share of the brand "Yunyao", and build Yunnan into a biomedical R&D innovation and product production and trade center with distinctive characteristics of serving the whole country and radiating South Asia and Southeast Asia, and build an industrial cluster with important influence in the country.
-do it on a large scale. By 2025, we will cultivate more than one enterprise (group) with operating income exceeding 50 billion yuan, more than five enterprises (groups) exceeding 10 billion yuan, more than five enterprises (groups) exceeding 5 billion yuan, more than 20 enterprises (groups) exceeding 1 billion yuan and more than 70 enterprises exceeding 100 million yuan.
-strengthen varieties. By 2025, more than 10 varieties with sales income exceeding 1 billion yuan and more than 60 varieties with sales income exceeding 100 million yuan will be cultivated.
-do an excellent structure. By 2025, the leading development of biotechnological drugs will be realized, modern Chinese medicine (ethnic medicine) will be upgraded and developed, dual-use products for medicine and food will develop by leaps and bounds, and chemical drugs and medical devices will make breakthroughs.
-fill in the short board. We will build and upgrade a number of public R&D and service platforms around biotechnology drugs, modern Chinese medicine and chemical drugs, break through a number of key core technologies, and develop a number of competitive and innovative products.
Third, key tasks
Around the biomedical industry chain, deploy innovation chain, promote the organic integration of industry chain and policy chain, innovation chain, capital chain, supply chain, talent chain and value chain, focus on key core technologies, fill the shortcomings of the platform and increase high-quality varieties. Strengthen leading enterprises and enhance manufacturing capacity. Optimize the industrial layout, strengthen the construction of the park, and form industrial agglomeration. Enhance the brand of "Yunyao", revitalize the stock, introduce increments, expand the total amount, and realize the high-quality development of the biomedical industry.
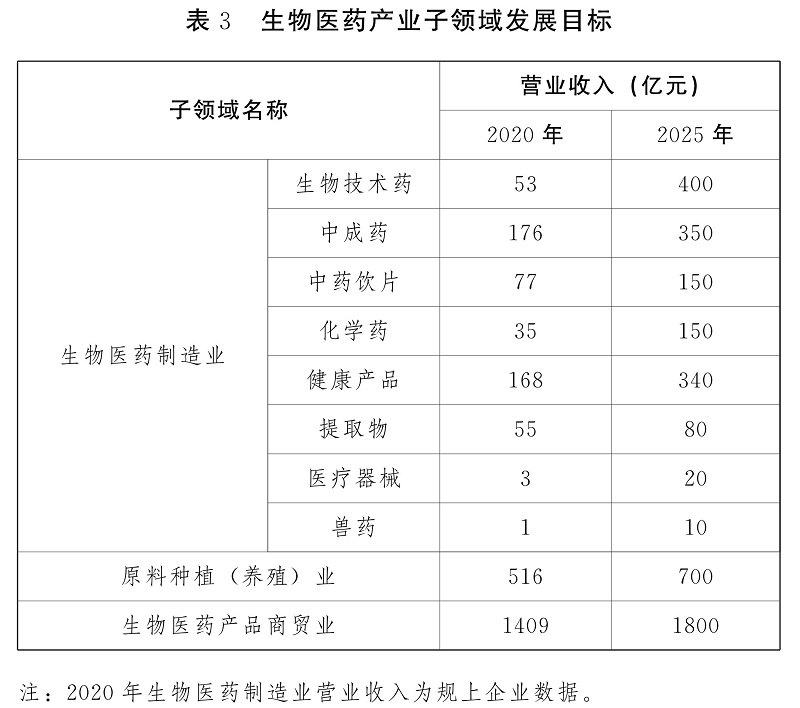
(1) Strengthen varieties and accelerate the transformation and upgrading of biomedical manufacturing.
Give priority to the development of biological vaccines, modern Chinese medicines (ethnic medicines), Chinese herbal pieces and chemical medicines, vigorously develop biotechnological medicines such as antibody medicines, gene technology, cell therapy and blood products, as well as health products, detection and diagnosis reagents, medium and high-end medical devices, strengthen the research and innovation of biotechnological medicines, modern Chinese medicines, natural medicines, generic medicines, new preparations and health care products, and support the internationalization of vaccines, modern Chinese medicines and medical devices. Focusing on increasing investment in scientific and technological innovation, building a number of well-known enterprises and brand products, introducing large enterprises and groups, encouraging enterprises to acquire and merge, and promoting international development, we will promote digital and intelligent manufacturing and accelerate the transformation and upgrading of biomedical manufacturing.
1. Key development areas
(1) Biotechnology drugs focusing on vaccines
The biotechnology pharmaceutical industry focusing on vaccines is the fastest-growing field in the biomedical industry in our province, and efforts should be made to improve the upstream and downstream industrial chains, enhance the research and development capacity of new vaccines, accelerate the construction of a rapid response system for major infectious diseases, strengthen frontier basic research, and enrich product pipelines.
Focus on Kunming and Yuxi to build biotechnology pharmaceutical industrial bases focusing on vaccines, and vigorously develop new biological vaccines, antibody drugs, biological analogues, cell preparations, blood products such as albumin, coagulation factors and immunoglobulin, and other new products of proteins, peptides and nucleic acids. Enlarge and strengthen the existing biotechnology drug enterprises, encourage vaccine enterprises to build digital and intelligent production lines, and improve the production technology level. Guide enterprises in the province to build, acquire and merge R&D institutions at home and abroad, build a quality system, R&D system and production technology system that are in line with international standards, promote the international registration and WHO pre-certification of biological vaccine products, build a vaccine production and supply base that meets international quality standards and a leading domestic monoclonal antibody drug industry base, increase open cooperation with South Asia, Southeast Asia, Africa, Europe and the United States, and open up markets in South Asia, Southeast Asia and the Indian Ocean Rim.
Traditional vaccines: enterprises are encouraged to develop univalent vaccines, multivalent vaccines and multiplex vaccines with attenuated, detoxified and inactivated products based on pathogenic microorganisms and their metabolites, so as to promote the secondary development and upgrading of biological vaccines.
New vaccines: break through the key core technologies such as efficient genetic engineering construction technology of virus species and strains, screening research technology of pathogen antigen components, bacterial polysaccharide-protein binding technology, large-scale animal cell culture technology, new vaccine adjuvant technology, genetic engineering technology, genetic engineering recombination technology, non-human primate model evaluation, and carry out research and development and transformation of new vaccines such as nucleic acid vaccine, synthetic peptide vaccine, genetic engineering vaccine and recombination vaccines. Increase the forward-looking research and development of mRNA technology in the prevention and treatment of infectious diseases, AIDS, genetic diseases, rare diseases, tumors and other major diseases.
Antibody, protein and nucleic acid drugs: develop monoclonal antibody drugs, neutralizing antibodies, bispecific antibodies, antibody-coupled drugs (ADC), biological analogues, recombinant protein drugs, polypeptide drugs and nucleic acid drugs for diseases such as tumor, immune system, cardiovascular and cerebrovascular diseases and infections.
Animal vaccines: Encourage the research and development of veterinary vaccines such as Class A epidemic vaccines, multi-animal co-morbid vaccines, vaccines for cattle and sheep, vaccines for pigs, vaccines for poultry, and vaccines for pets.
Cell preparation: within the scope permitted by policies and regulations, encourage and standardize the research and development of cell therapy products including stem cells and immune cells; We will carry out research on a series of standards and technical systems such as preparation, quality inspection and safety evaluation of clinical-grade cell products and derivatives, improve their safety, effectiveness and quality controllability, and accelerate the research on clinical application norms of cell therapy.
Blood products: introduce and develop blood products, focusing on the development of coagulation factors, thrombin, human albumin, human fibrinogen, immunoglobulin and other products. Within the scope permitted by the policy, the layout of plasma raw materials will be increased by adding plasma collection stations.
Other biotechnologies: Accelerate the construction of a synthetic biotechnology innovation system, support the research on genome design and synthesis, and provide technical support for basic research on new biological treatment methods, vaccines, materials and disease control.
Make use of the advantages of genetic resources in our province to develop the biomedical industry of non-human primates and carry out research on the protection and rational utilization of human genetic resources; Carry out research on transplant organ cultivation technology and xenotransplantation technology.


(2) Modern Chinese medicine represented by formula granules.
Focus on the quality upgrade, secondary development, new indications mining, economic evaluation, clinical value-oriented research on safety and clinical efficacy evaluation of traditional Chinese medicine, and innovative drug development of traditional Chinese medicine, and make efforts in the research on local standards of characteristic Chinese herbal pieces, quality standards that meet the requirements of Pharmacopoeia, effectiveness and safety evaluation, production process improvement, and automation, intelligence and information construction of production and processing.
Focus on Kunming, Yuxi, Chuxiong, Dali, Wenshan, Baoshan, Pu ‘er, Honghe and other cities to develop modern Chinese medicine. On the basis of medicinal animal and plant resources such as Panax notoginseng, Erigeron breviscapus, Gastrodia elata, Paris polyphylla, Periplaneta americana, leech, etc., and taking the development of characteristic ethnic medicines such as Yi medicine and Dai medicine as a breakthrough, we will speed up the cultivation of large varieties of modern Chinese medicine (ethnic medicine) preparations, create a number of brand products, and make Yunnan medicine industry bigger and stronger. Based on classic prescriptions, proven prescriptions and hospital preparations, we will develop innovative Chinese medicine (ethnic medicine) and epidemic prevention preparations covering the whole life cycle of prevention, treatment and rehabilitation for the prevention and treatment of major diseases such as malignant tumors, cardiovascular and cerebrovascular diseases, emerging infectious diseases and AIDS. Guided by clinical value, we will carry out material basic research, safety evaluation of traditional Chinese medicine and clinical efficacy research, and explore the construction of a three-in-one evidence system of "Chinese medicine theory-human experience-clinical trial" for the research and development of new traditional Chinese medicine. Adopt modern biotechnology, advanced pharmaceutical technology and preparation technology to promote the application of biocatalysis and transformation, refining, extraction and separation in the research and development of modern Chinese medicine.
In Kunming, Chuxiong, Wenshan, Zhaotong, Pu ‘er, Baoshan, Lijiang and other major producing areas, the production base of Chinese herbal pieces will be laid out, with the focus on building Chuxiong into a national first-class industrial base of Chinese herbal formula granules. Form more than 10 brand enterprises producing Chinese herbal pieces and local standards for formula granules of more than 100 varieties. Strengthen cooperation with state-level research institutions and well-known domestic enterprises, carry out research on quality standards of traditional Chinese medicine formula granules and upgrade the processing and production technology, and establish a quality standard system, a safety evaluation system and a clinical trial research system for traditional Chinese medicine decoction pieces that meet the requirements of People’s Republic of China (PRC) Pharmacopoeia and international standards. Carry out the equivalent test and production technology research of formula granules, and establish the processing technology and quality evaluation method of traditional Chinese medicine decoction pieces combined with modern science and technology. Develop new modern technologies, equipment and facilities such as extraction, concentration, drying and granulation, apply blockchain traceability technology to improve online quality control, establish a traceability system for processing and production of Chinese herbal pieces, and build a number of intelligent production lines of Chinese herbal pieces with advanced technology and strict quality control.
We will build a research center for ethnic medicine focusing on Yi medicine, Dai medicine and Tibetan medicine, systematically excavate and sort out ethnic medicine documents of Yi, Dai, Tibetan, Naxi, Wa and Hani nationalities, and carry out clinical application of traditional ethnic medicine therapy and its ancillary products and theoretical research on ethnic medicine. Relying on ethnic medicine resources, develop external preparations. Strengthen the research and development and clinical application of preparations in ethnic medicine hospitals, and carry out research and development of new ethnic medicine with clear mechanism, high technology content and reliable curative effect.
Build a characteristic industrial cluster of Sanqi, and build Sanqi into the first brand of traditional Chinese medicine in China. We will build a Sanqi industrial innovation consortium with enterprises as the main body, and gather superior resources inside and outside the province to carry out key technology research and transformation and application of achievements. Develop innovative drugs for prevention, treatment and rehabilitation of cardiovascular and cerebrovascular diseases; Focusing on the large varieties and exclusive varieties of traditional Chinese medicine (ethnic medicine) such as Sanqi series and Dengzhanhua series, we will increase the re-evaluation after listing and the secondary development of "new use of old drugs", and carry out research on the combination of traditional Chinese and western medicine such as "Xuesaitong+aspirin"; Strengthen the biosynthesis and application of panax notoginseng saponins, scutellarin, Paris polyphylla saponins and other extracts; Carry out research on the processing technology and technology of traditional Chinese medicines such as Panax Notoginseng while fresh, and formulate standards for new-type decoction pieces such as formula granules and powder decoction pieces of characteristic varieties such as Panax Notoginseng and Gastrodia elata which meet the standards of People’s Republic of China (PRC) Pharmacopoeia; To study and formulate EU Pharmacopoeia standards for Sanqi formula granules, panax pseudo-ginseng and other products, so as to enhance the international popularity and influence of Sanqi.
Regional cooperation in the research and development of traditional Chinese medicine (ethnic medicine) should be carried out for South Asia and Southeast Asia, international multi-center clinical trials and international registration of traditional Chinese medicine preparations with good clinical effect and clear mechanism of action should be carried out, and a modern standard production system and quality evaluation system of traditional Chinese medicine should be constructed according to international standards.

(3) Health products based on natural extracts.
Continue to strengthen the research on the material basis of effective active ingredients of animals and plants, increase the development and application of extracts, strengthen the development of high-quality health products, and cultivate brand products with market competitiveness and scale effect.
Focus on Kunming, Yuxi, Chuxiong, Pu ‘er, Xishuangbanna, Lijiang, Wenshan and other cities to build health product research and development and production bases, and develop high value-added health products. Focus on health care products and daily chemical products such as Sanqi series, Dendrobium series and microalgae series, and build a number of brand products with sales income exceeding 1 billion yuan. Introduce well-known enterprises at home and abroad, cultivate some leading enterprises, and promote Yunnan’s health products to South Asia and Southeast Asia.
Construction of Yunnan laboratory for extracting characteristic plants. Focusing on plant extraction and scientific and technological innovation in the whole industrial chain of health products, we will systematically carry out basic research, product development and achievement transformation, and build a platform for functional evaluation and inspection of health foods and cosmetics. Using modern biotechnology, we will strengthen the research on the efficacy and application of animal and plant-derived ingredients, cultivate and expand a number of intensive processing backbone enterprises in the main raw material producing areas in view of the needs of food, medicine, health care products, daily chemical products, feed products and other related industries, and promote the extraction and processing of characteristic Chinese herbal medicines such as Panax notoginseng, Periplaneta americana and leech, as well as the raw materials of characteristic health products such as stevia rebaudiana, eucalyptus globulus, marigold, maca, rose, microalgae and aloe, and research and development. Improve the extraction and separation technology and quality level of panax notoginseng saponins, breviscapine, tea polyphenols, coenzyme Q10, paclitaxel, borneol and other extract products, as well as eucalyptus oil, citronella oil, rose oil, rosemary and other essence and fragrance products. Taking notoginseng, gastrodia elata, Dendrobium nobile, Polygonatum sibiricum, Amomum villosum and other medicinal and edible Chinese herbal medicines as raw materials, and special biological resources such as microalgae, Moringa oleifera, walnuts and flowers as raw materials, we have researched and developed various health products such as medicated diet, medicated wine, medicated tea and drinks, as well as a series of health foods, aromatic products and daily necessities with the functions of resisting oxidation, losing weight, enhancing immunity, relieving anxiety, relieving depression, assisting in improving memory and caring skin. Focus on developing and producing a batch of formula foods with strong pertinence and high patient acceptance, which are suitable for surgery, nephrology, oncology, pediatrics and geriatric rehabilitation.
Improve the development level of Panax notoginseng extract. In-depth study of extracts from different parts such as flowers, stems, leaves, roots, etc., and development of Sanqi series health food, special diet products, oral care, medical beauty skin care and other daily necessities in different dosage forms such as tablets, capsules and oral preparations, enriching product structure and expanding application fields; Improve the extraction technology and product quality level of panax notoginseng saponins, polysaccharides and panax notoginseng elements; Study and formulate international standards for the extraction of characteristic raw materials such as Panax notoginseng, and accelerate the internationalization of Panax notoginseng.


2. Rapid development areas
(1) Chemical drugs
Further improve the industrial chain, improve the research and development capabilities of original drugs and generic drugs, and improve the research and development level of high-end preparations.
Focus on Kunming, Chuxiong and other cities to build R&D and production bases for chemical drugs and chemical raw materials, and carry out R&D and industrialization of innovative drugs, improved new drugs, generic drugs and chemical raw materials.
Strengthen the joint development of "intermediates+APIs+chemicals". Improve the production technology of platinum anti-tumor raw materials and steroid hormone raw materials. Encourage enterprises to buy varieties with high clinical value at home and abroad and enterprises with great development potential to enter Yunnan for development. Develop chemical raw materials, auxiliary materials and packaging materials industries in areas with resources and environmental carrying capacity, and develop a number of chemical synthetic drugs with characteristics, potential and competitiveness. Strengthen exchanges and cooperation with Israel, India and other countries in the research and development of generic drugs, and explore the construction of an international cooperation base for the research and development of generic drugs.
Guide key enterprises and scientific research institutions to focus on the development of innovative drugs for major diseases such as malignant tumors, cardiovascular and cerebrovascular diseases, diabetes, mental diseases, viral infections, etc., especially chemical innovative drugs and improved new drugs with new targets and new mechanisms of action. Carry out research and development of high-end preparations, develop new dosage forms with good clinical substitution for varieties and dosage forms that are clinically necessary and have great side effects, and promote the research and development of sustained and controlled release, targeted, transdermal, mucosal and carrier drug delivery systems. Develop Yunnan plant resources, discover and develop new natural chemical drugs. To carry out the imitation of drugs needed for the treatment of major infectious diseases and rare diseases, drugs needed for the disposal of public health emergencies, drugs used by children, and original drugs whose patents are about to expire. Carry out research and development and green production technology innovation in the fields of chemical raw materials and pharmaceutical intermediates; Carry out the construction of the process technology system for the biosynthesis of important pharmaceutical monomer compounds such as panax notoginseng saponins and breviscapine.
(2) Medical devices
Give full play to the advantages of China (Yunnan) Pilot Free Trade Zone (hereinafter referred to as FTZ) and Border Economic Cooperation Zone, speed up the construction of medical device production and trade system for South Asia and Southeast Asia, develop high-tech and high value-added products, and establish an effective linkage mechanism between medical institutions and medical device industry.
We will build medical device industrial parks in Central Yunnan New District, Qujing, Yuxi, Free Trade Zone and Border Economic Cooperation Zone, build medical device R&D and production, inspection and testing, and trade distribution bases for South Asia and Southeast Asia markets, and build the most complete medical device industrial park in Southwest China.
In view of the market demand in South Asia and Southeast Asia, we will introduce domestic and foreign leading or advantageous enterprises in high-end medical devices and biomaterials to build medical device production bases and supporting industrial chains, cultivate high-end medical device enterprises, and promote the development of upstream and downstream industries of medical devices. Support the construction of a production base that meets the quality management standards for medical device production, build a public R&D and production platform for medical devices, build a high-grade medical device life-cycle detection technology service platform based on units with good foundation, and attract enterprises, research institutions, talent teams and technological achievements to Yunnan to carry out the transformation and industrialization of achievements. We will build a number of leading enterprises in the production of in vitro diagnostic reagents, dentures and disposable infusion devices, and promote the development of industries such as medical devices, new biomaterials, high-end pharmaceutical packaging materials and high-quality pharmaceutical excipients.
Develop technologies and products such as gene detection, biological detection chip and in vitro immunodiagnosis, and develop in vitro diagnostic equipment and reagents such as major emerging infectious diseases and tumor diseases; Accelerate the research and development of new biomaterials, and carry out research and development of new biomaterials such as biomedical materials, tissue engineering materials, bio-based materials and medical and health materials; Carry out research and development and production of high-end consumables such as orthopedic instruments, and build a production base of high-end orthopedic consumables in southwest China; Carry out research and development of TCM diagnosis and treatment instruments and equipment, rehabilitation instruments and equipment, and sports therapy equipment with ethnic characteristics; Carry out the development and industrialization of "big data+artificial intelligence+medical care" instruments and equipment, and develop digital and multifunctional mobile monitoring equipment and wearable products, rehabilitation AIDS and optometry-related products.
(3) Veterinary drugs
Further guide enterprises and scientific research institutions, strengthen investment in veterinary drug research and development, enhance the research and development ability of veterinary drugs, and give full play to the advantages of resources to develop innovative products of veterinary drugs.
Strengthen the cooperation between animal medicine enterprises and scientific research institutions inside and outside the province, and develop or introduce a new generation of animal vaccines and innovative products for veterinary medicine. Improve the quality and production capacity of a number of veterinary drugs, such as bovine O-type and A-type foot-and-mouth disease inactivated vaccine, pig O-type foot-and-mouth disease inactivated vaccine, pig double O-type foot-and-mouth disease inactivated vaccine, hog cholera live vaccine (subculture cell source), piglet paratyphoid, Newcastle disease and so on.
Focus on Kunming, Baoshan, Dali and other cities with veterinary drug industry base to actively develop veterinary drug industry. Increase the research and development of livestock and poultry disease prevention vaccines and pet vaccine products such as cattle, sheep, pigs and chickens; Develop a batch of veterinary traditional Chinese medicine preparations with traditional Chinese medicine as raw materials, which can replace antibiotics, carry out veterinary drug imitation research, develop feed drug additives, improve the self-sufficiency rate of veterinary drug production, and actively expand the market in South Asia and Southeast Asia.

(2) To improve quality and accelerate the construction of high-quality raw material bases for Chinese herbal medicines.
Highlight the construction of standardized planting (breeding) bases for Chinese medicinal materials, adhere to the principle of quality first, control the planting scale, innovate the planting mode, promote the original habitat planting, improve the quality of medicinal materials, and scientifically develop large varieties of raw materials such as Panax notoginseng, Erigeron breviscapus, Paris polyphylla, Gastrodia elata and Dendrobium. At the same time, give play to ecological advantages, actively introduce domestic and foreign high value-added medicinal varieties into Yunnan to build a high-quality raw material planting and processing base. Make good use of domestic and foreign resources and markets to build Yunnan into a national raw material base for high-quality natural medicines and health products.
1. Breeding excellent provenances and developing the fine variety industry of Chinese herbal medicines.
In order to promote the sustainable development of Chinese herbal medicine resources, the collection and evaluation of germplasm resources, screening of high-quality provenances, breeding of new varieties and breeding of improved varieties will be carried out around the backbone medicinal materials and rare and endangered Chinese herbal medicines, and the industry of improved varieties of Chinese herbal medicines will be developed. In view of the problems such as source degradation, we will strengthen the domestication and planting of Chinese herbal medicines and the breeding of improved varieties. Strengthen the investigation, collection, identification, preservation and artificial domestication and cultivation techniques of important rare and precious wild raw material germplasm resources such as Fritillaria cirrhosa, Euphorbia Humifusa, Rhizoma Curculiginis, Rhubarb and Dragon’s Blood, and protect the germplasm and genetic resources. In Wenshan, Zhaotong, Chuxiong, Dali, Lijiang, Pu ‘er, Baoshan and other authentic Chinese herbal medicine producing areas and suitable planting (breeding) areas, 50 breeding bases of authentic superior Chinese herbal medicines, 100 guaranteed nursery bases of Chinese herbal medicines and 100 standardized planting (breeding) bases will be upgraded, and a number of Chinese herbal medicine seed and seedling enterprises will be built, with emphasis on cultivating more than 10 high-quality seed and seedling management companies. Relying on the National Nature Reserve, conservation zone, a Chinese herbal medicine, will be built, and more than two protected areas for endangered and rare wild medicinal plants and animals will be planned, and more than 10 wild breeding bases for endangered and rare genuine medicinal materials from Yunnan will be built to provide support for the rapid development of raw materials industry for health products.
2. Strengthen the construction of high-quality raw material bases and build high-quality Chinese herbal medicine planting areas.
Build the "first workshop" of biomedical industry with the concept of green development. According to GAP standards, develop rural areas to revitalize the "one county, one industry" Chinese herbal medicine planting (breeding) industry. Innovate the planting (breeding) mode and vigorously develop "green planting", "ecological planting", "under forest planting" and "imitation of original habitat planting" of Chinese herbal medicines. Encourage domestic and foreign enterprises to build a "pharmaceutical park" in suitable areas of Yunnan, and accelerate the formation of a situation in which leading enterprises lead, bases are standardized and the province develops in an orderly manner. Cultivate a number of well-known enterprises and high-quality raw material varieties, and build a standard library of reference materials for Chinese herbal medicine varieties. Cooperate with neighboring countries to protect and organize traditional medicinal resources in South Asia and Southeast Asia, popularize planting techniques and carry out overseas planting. Strengthen the research and development and application of advanced equipment for planting Chinese herbal medicines, and improve the modernization level of planting (breeding) Chinese herbal medicines. Cultivate original bulk and regional comprehensive primary processing bases of Chinese herbal medicines, and lay out and build regional inspection and testing platforms.
Give full play to the advantages of Yunnan’s ecological environment, national culture and tourism industry, and promote the construction of a number of Chinese herbal medicine health industrial parks (manors) including standardized cultivation (breeding), food culture, health experience, leisure and health preservation, and popular science propaganda, with emphasis on building more than 10 well-known manors.
3. Accelerate the improvement and efficiency of "the hometown of Yunnan medicine" and build the brand of "Yunnan medicine"
Relying on the "Hometown of Yunyao", we will promote the construction of more than 60 standardized, large-scale and standardized planting (breeding) areas of high-quality Chinese herbal medicines, improve the standards of authentic Chinese herbal medicines, study and formulate a number of domestic and international standards for raw materials of health products, expand the scale of green planting and ecological planting of Chinese herbal medicines, standardize the whole process management of raw material planting (breeding), and build a quality monitoring network and traceability system covering the main producing areas of Chinese herbal medicines in the province. Build a green production technology promotion system, a production processing technology system, a quality evaluation system and a traceability system of the whole industry chain, and build a "Top Ten Yunyao" brand. We will carry out basic research on pharmacology, medicalization, efficacy and safety evaluation of Chinese herbal medicines, and develop a number of new food raw materials.
Build Sanqi into a national demonstration variety of Chinese herbal medicine intelligent agriculture. Improve the technology and level of green planting, undergrowth planting and original habitat planting of Panax notoginseng, and improve the supply capacity of high-quality and high-end products. Around the breeding, planting, processing, warehousing and other links of improved varieties of Panax notoginseng, we will build a comprehensive and domestic leading big data platform for intelligent agriculture of Panax notoginseng, and build a digital management and service system for seed and seedling quality evaluation, monitoring and early warning of pests and diseases, intelligent diagnosis, processing of origin, warehousing and logistics, quality inspection, product traceability and so on.

(three) to expand the market and accelerate the improvement of the business system of biomedical products.
Based in Yunnan, facing the whole country, South Asia, Southeast Asia and the Indian Ocean Rim, with the help of internet plus, big data, cloud computing and other modern technical means, we will give full play to the role of free trade zones and border economic cooperation zones, vigorously carry out internal and external cooperation, and build a business system for biomedical products with the focus on market system construction, enterprise management informationization and international business development.
1. Build a regional circulation system
Focusing on free trade zones and border ports, we will lay out and build a number of modern warehousing and logistics centers for biomedical products in all provinces, cities and key counties, cities, districts and border port cities such as Hekou and Ruili, build regional pharmaceutical wholesale and distribution centers, build cold chain logistics, and improve the supply guarantee system for pharmaceutical and health products in Yunnan. High-level construction of Yunnan Chinese herbal medicine trading center, enlarge and strengthen Wenshan Sanqi, Zhaotong Tianma and other regional characteristic Chinese herbal medicine markets, build a futures market for bulk medicinal materials and scarce medicinal materials, and cultivate a regional distributed trading market for fresh medicinal materials. Strengthen the supervision of Chinese herbal medicine market, standardize the access management of Chinese herbal medicine market, and establish a quality inspection, public warehousing and quality traceability system.
2. Promote the development of new logistics formats
Promote the "internet plus" business model, encourage drug circulation enterprises to use the Internet and Internet of Things technology, introduce third-party service organizations such as insurance and logistics, provide extended services such as online pharmacies and drug distribution, and build an information management system covering the whole province’s business circulation. Taking the Chamber of Commerce as the main body, we will jointly build the "Yunyao Information Network" and "Yunyao Database" to build the Yunnan pharmaceutical e-commerce trading platform. Develop the platform economy of "internet plus Traditional Chinese Medicine+Traditional Chinese Medicine+Health Service" and build a new format and new model economy of Chinese medicine industry.
3. Broaden the market channels of Yunyao.
Strengthen cooperation in commercial production, encourage commercial circulation enterprises inside and outside the province to sell Yunnan products, guide commercial circulation enterprises to build production and marketing alliances with raw material planting enterprises and pharmaceutical and health care products production enterprises, and provide market information and sales channels for production enterprises. Develop a number of specialized institutions that exchange and cooperate with domestic and foreign countries, and hold various seminars, exchanges and exhibitions. Encourage business circulation enterprises in the province to become bigger and stronger, and lead production enterprises, especially small and medium-sized enterprises, to expand domestic and foreign markets.

Fourth, the main measures
(1) Vigorously implement accurate investment promotion.
In-depth implementation of the "multiplication" plan of market players, focusing on biotechnology drugs, modern Chinese medicine, chemical pharmaceuticals, health products and other fields, aiming at the top 500 international companies, the top 100 biomedical companies and leading enterprises in the industry, and carrying out accurate investment promotion. Implement the drug marketing license holder system, and introduce innovative achievements holders such as biotechnology drugs, innovative Chinese medicines and chemical medicines to Yunnan to carry out achievements transformation and industrialization. Introduce and develop serum-free cell culture medium, affinity filler medium, ultrafiltration membrane, disposable reaction bag and other upstream and downstream supporting enterprises of biological products represented by vaccines to enter Yunnan for development. Introduce Chinese herbal pieces and health products processing enterprises represented by formula granules into Yunnan to build a raw material base, research and development production base and extend the industrial chain of Chinese herbal medicines. Implement the medical device holder system, give full play to the advantages of free trade zone and border economic cooperation zone, introduce medical device R&D and production enterprises to Yunnan, and build a medical device production and circulation base for South Asia and Southeast Asia. Introduce chemical raw material medicine enterprises into Yunnan for development in areas with resources and environmental carrying capacity. In combination with the introduction of science and technology into Yunnan, we will actively promote the "four landings" of talent teams, scientific and technological enterprises, scientific and technological achievements and innovation platforms, and strengthen the introduction of CRO (contract research and development institutions) and CMO (commissioned production institutions).
(2) Accelerate major projects.
Implement special projects for the development of biomedical industry, organize and implement a number of key projects to support and lead the development of biomedical industry. Relying on leading enterprises and R&D institutions with strong R&D capabilities, we will organize and plan a number of major projects combining "Industry-University-Research" for the development needs of key areas of biomedicine. With the goal of leading enterprises and key brand products becoming bigger and stronger, we will implement a number of quality improvement and efficiency improvement projects through digital and intelligent technological transformation and secondary development. With the goal of building industrial clusters, support key cities, key parks and key enterprises to plan and implement a number of key projects. Encourage the park to plan a number of preliminary projects and implement a number of key projects around infrastructure construction, key industrial bases construction and major scientific research platforms construction, and strengthen tracking management and service guarantee.
(C) focus on cultivating leading enterprises
Implement the cultivation plan of leading enterprises, and comprehensively improve the comprehensive competitiveness of pharmaceutical enterprises. In accordance with the principle of "one thing, one discussion, one enterprise, one policy", we will enlarge and strengthen leading enterprises, cultivate a number of large enterprise groups with international influence, develop and expand a number of key enterprises, support the development of small and medium-sized enterprises, and form enterprise clusters. In the fields of traditional Chinese medicine, biotechnology medicine, chemical medicine and health products, select a group of enterprises with good growth to focus on cultivation. Support enterprises to build raw material bases and production bases around the industrial chain, and improve the level of automation, intelligence and informatization of enterprises. Carry out the listing cultivation of biomedical enterprises, and continue to promote the listing progress of biomedical "golden seed" enterprises. Support enterprises to take capital, technology, brand as the link, through mergers and acquisitions, agreement transfer, joint restructuring, holding shares and other ways to carry out mergers and acquisitions, improve industrial concentration.

(4) Continuously improve the innovation capability.
Focusing on the fields of vaccines, antibody drugs, cell products, modern Chinese medicine, formula granules, chemical drugs, health products, extracts, etc., we will upgrade and build a number of industrial public service platforms and specialized research and development platforms, form a perfect biomedical research and development and technical service chain, and continuously improve the ability of biomedical innovation. Keep a close eye on the commanding heights of industry technology, increase investment in applied basic research, and reserve a number of advanced and applicable technologies and achievements; Adopt the innovative project organization mode of "unveiling the list", aim at the major needs of the industry, break through key technologies, formulate new standards, develop new products and upgrade new processes. With enterprises as the main body, we will build a number of R&D and service platforms such as key laboratories, technology innovation centers, engineering research centers, enterprise technology centers and industrial technology innovation platforms in conjunction with well-known expert teams and scientific research institutions at home and abroad. Promote the construction of the State Key Laboratory of Biomedicine for Non-human Primates, accelerate the construction of Yunnan Laboratory for Extraction of Characteristic Plants, build a new vaccine technology innovation system, and improve the research platforms such as natural drug screening, research on patent medicine, drug safety evaluation (GLP), drug pilot test, drug clinical evaluation platform (GCP) and bioequivalence test. Construction of provincial clinical medical research centers and a number of national clinical medical research sub-centers. Cultivate and introduce several CRO institutions. Promote and build a platform for big data and digital applications around the biomedical industry chain.

(5) Building professional parks with high quality.
With Kunming as the center and Yuxi, Chuxiong, Wenshan, Qujing, Baoshan, Honghe and other cities as the focus, a number of biomedical industry specialized parks covering the whole province will be built. Focus on promoting Kunming National Bio-industrial Base, Yunnan Central Traditional Chinese Medicine Natural Medicine Economic Circle, Yunnan Central Medical Device Industrial Park, Kunming and Yuxi Bio-vaccine R&D and production bases, International Medical Health City, Chuxiong Traditional Chinese Medicine Formula Granule Industrial Base, Wenshan High-tech Industrial Development Zone, Tengyao Industrial Park, Pu ‘er Biomedical Industrial Park, Honghe Biomedical Industrial Cluster, Qujing Malong Medical Device Emergency Industrial Park and other specialized parks and clusters. Based on the evaluation of the carrying capacity of resources and environment and the suitability of land and space development, chemical raw material medicine parks will be developed in Kunming, Chuxiong and other areas that meet the relevant policy requirements of chemical parks. Promote the gathering of projects, funds, talents and other elements in the park, strengthen the construction of public R&D and service platform, industrial incubation, infrastructure, industrial facilities and human resources conditions in the park, and form a regional specialized park integrating research and development, enterprise incubation, manufacturing and logistics distribution. Strengthen cooperation with biomedical industrial parks in Beijing-Tianjin-Hebei, Yangtze River Delta, Pearl River Delta and other regions, attract large domestic and foreign pharmaceutical enterprises, scientific research institutions and teams, CRO institutions and CMO institutions to settle in the parks, promote industrial agglomeration development, vigorously promote the construction of Kunming Great Health Industry Demonstration Zone, and build Kunming and Yuxi into important bases and agglomeration areas for modern biomedical production and research, and Chuxiong, Wenshan and other cities into important bases for modern Chinese medicine industry.
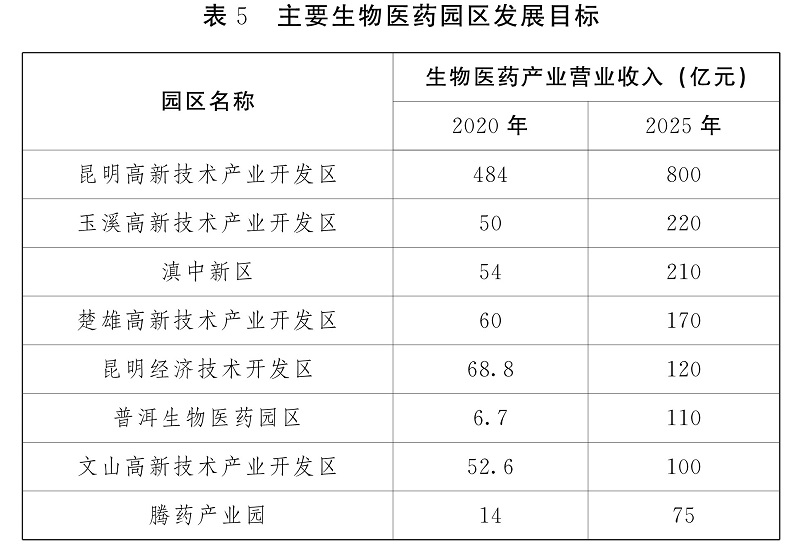
(6) Accelerate the training and introduction of talent teams
Adhere to the problem orientation, deploy the talent chain around the industrial chain and innovation chain, promote all kinds of talent training and introduction programs to the biomedical industry, further strengthen the training and introduction of high-level talents, and introduce high-end management talents, leading scientific research talents, high-level technical talents and innovative teams through multiple channels. Innovate training methods, train all kinds of talents, expand the enrollment scale of biomedical related majors in colleges and universities, and establish specialized talent training bases relying on qualified colleges and universities. Encourage social capital to set up vocational colleges and training institutions, promote school-enterprise cooperation, jointly cultivate and improve the quality of employees, and focus on cultivating a number of scientific and technological research and development talents, enterprise management talents, marketing talents, licensed pharmacists, quality management, testing and consulting service talents. Encourage the park to set up special funds for talents to provide more powerful talent support for the high-quality development of biomedical industry.
(7) Focus on building the brand of Yunyao.
Focus on the superior products such as vaccine series, Sanqi series and Dengzhanhua series, and build the brand of "Yunyao". To build a biological vaccine brand, we should build a specialized vaccine industry park, build a vaccine research and development system, develop innovative vaccine products, and promote the international development of vaccines. Extend the industrial chain of large varieties of Chinese herbal medicines such as Panax notoginseng, Erigeron breviscapus, Paris polyphylla, Dendrobium, Gastrodia elata, cultivate a number of large varieties of Chinese herbal medicines, and create geographical indication protection varieties. Develop ethnic resources of traditional Chinese medicine, develop and expand a number of dual-purpose varieties of traditional Chinese medicine, and promote the formation of a number of brand products such as Chinese herbal pieces, extracts, health foods and functional cosmetics. Implement the promotion action of famous Yunnan medicine products, give play to the leading role of the propaganda department, and form a publicity system with the participation of the government, enterprises and social organizations. Encourage enterprises to carry out various forms of publicity and promotion activities to publicize the advantages, characteristics, achievements, enterprises, brands, products and people of Yunnan’s development of biomedical industry. Build a brand display platform through various trade fairs such as South Expo and Drug Fair.
V. Guarantee conditions
(A) to strengthen organizational leadership
Improve the bio-pharmaceutical industry promotion mechanism, compact work responsibilities, hold regular meetings, analyze industrial development, study industrial policies, deploy promotion work, coordinate and solve key problems in the development of key enterprises and major projects, and promote vertical linkage at the provincial, prefecture and county levels. Further improve the horizontal linkage mechanism of provincial departments, enhance the joint efforts of all departments to promote the development of biomedical industry, and jointly create a good environment to promote the high-quality development of biomedical industry in Yunnan. An expert advisory committee on biomedical industry composed of senior experts in relevant fields inside and outside the province was established to provide strategic advice on the frontier situation, industrial analysis, innovation ability, regional layout, key investment promotion and policy design of Yunnan biomedical industry.
(2) Increase policy support.
Focusing on the research and innovation of new products, achievements transformation, market development and innovative service system construction in the fields of biotechnology drugs, modern Chinese medicine, chemical drugs, medical devices and health products, we will coordinate financial funds, increase investment and refine specific measures to support the innovation and development of biomedical industry. To obtain the national major new drug creation science and technology projects, the national key research and development plan related key projects, according to the law and regulations to give supporting support. Support for key core technology research and key new product development, comprehensive use of open competition, publicity system, horse racing system, directional selection and other ways to organize major innovation projects. Study and formulate policies and measures to support the development of biomedical industry in the free trade zone, and help Yunnan biomedical industry to go out and introduce. Incorporate key projects and major projects involving industrial development into relevant national development plans and strive for national policy support. Actively strive for the inclusion of superior and characteristic drugs in the national basic medical insurance drug list and the national basic drug list. Encourage all localities to adopt the form of "government subsidies, enterprises making profits and people buying" to promote the project of biological vaccines benefiting the people. Deepen the "streamline administration, delegate power, strengthen regulation and improve services" reform and promote the development of industrial agglomeration.
(3) Overall factor guarantee
Through scientific planning, revitalizing stocks, introducing increments and other ways, we will further expand the development space of biomedical industry, and encourage industrial parks to build standardized factories to meet the needs of biomedical R&D and production enterprises on the basis of meeting the requirements of land and space planning and environmental assessment at all levels. Overall balance of land use, electricity consumption, water consumption, sewage and other indicators, scientific planning and layout of biopharmaceutical and innovative drug production bases. Encourage the park in accordance with the principle of intensive land use, overall planning for the protection of the park urgently needed talent introduction, employment units or park employees accommodation construction land. Focus on advantageous fields and varieties, establish key enterprises and project libraries, give preferential policies to key projects, and streamline examination and approval procedures according to laws and regulations.
(D) Expand financing channels
Give full play to the role of provincial financial control group industrial investment fund, and guide key cities and parks to set up biomedical industry investment funds. Strengthen cooperation with various investment companies, banks and other financial institutions, and adopt various ways and mechanisms such as listing financing, equity investment, credit guarantee, risk compensation, bond financing and asset management to promote base construction, product development, achievement transformation and industrialization, enterprise cultivation, brand building and market expansion.
(5) Strengthening digital empowerment
Promote the digitalization of resources, digital industrialization and industrial digitalization in the biomedical field. Apply modern information technologies such as blockchain, big data and artificial intelligence to upgrade the construction of Yunnan biomedical industry big data center, and further improve the production, supply and marketing network of biomedical enterprises, Chinese herbal medicine bases and circulation enterprises covering the whole province. Promote the construction of Yunnan biological resources digital integration center to realize the sharing, trading and calculation of biological resources scientific data. Give play to the role of industrial big data platform, promote data sharing in R&D, production, approval, sales and use, and break down departmental data barriers.
(6) Pay attention to safety and security.
Strengthen the construction of biosafety risk prevention and control and governance system, strengthen the supervision and management of high-risk and medium-risk biotechnology research and development activities, and take effective countermeasures. Give full play to the role of Yunnan Science and Technology Ethics Committee, strengthen overall planning and guidance, strengthen scientific research ethics supervision, standardize all kinds of scientific research activities, strengthen safety training for managers and professional and technical personnel, and ensure the safe and healthy development of biotechnology. We will comprehensively promote the construction of drug safety regulations and standards system, highlight the improvement of supervision efficiency, enhance the ability of drug monitoring and evaluation, audit and inspection, inspection and testing, and intelligent supervision, and realize the mutual promotion of safety supervision and industrial development. Combine the construction of the vanguard of ecological civilization construction, implement the concept of green development, strengthen environmental protection during the development and application of biotechnology products, and ensure ecological security.
(7) Strengthen evaluation and supervision.
Establish a dynamic evaluation mechanism for the implementation of the plan, formulate a task list every year, and regularly supervise the implementation of the plan by the relevant departments of the state, city and province. We will build a supervision and service mechanism for the operation of key enterprises and the construction of major projects, strengthen the operation monitoring of key enterprises and the scheduling, tracking and service of key projects, and promote the early commencement, completion and commissioning of projects.
Attachment: 1. Atlas of Yunnan Biomedical Industry Chain
2. Regional distribution map of "hometown of Yunyao"
3 main indicators, main tasks and key work responsibilities.
Annex 1
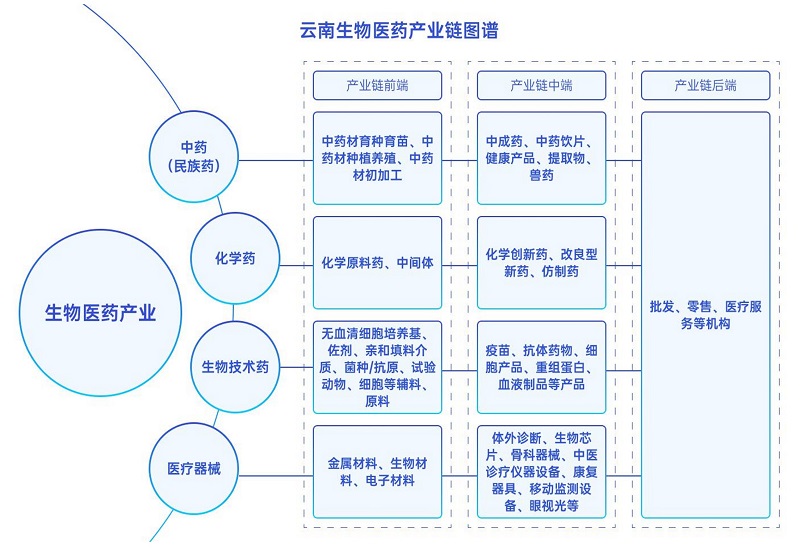
Annex 2
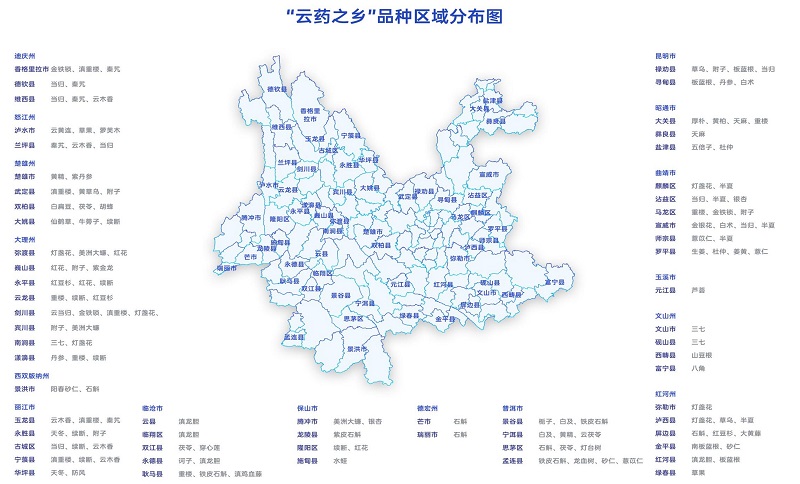
Annex 3